Introducción
El propósito de este Estudio fue examinar si la L-leucina (Leu), β-hidroxi-β-metilbutirato (HMB), o creatina monohidrato, evitaría posibles efectos atróficos provocados por la miostatina en el musculo esquelético. Para todo aquel que no sepa que función tiene la miostatina, la proteína miostatina (formalmente conocida como factor 8 de crecimiento y diferenciación) es un factor de crecimiento que limita el crecimiento del tejido muscular, por ejemplo concentraciones elevadas de miostatina en un individuo provocan una disminución en el desarrollo normal de los músculos. La proteína miostatina se produce en células del músculo esquelético, circula en sangre y actúa en el tejido muscular, al parecer retrasando el desarrollo de las células madre musculares. El mecanismo exacto sigue siendo desconocido. Hasta 2005, no hay drogas en el mercado que inhiban la Miostatina para los seres humanos, pero fue desarrollado un anticuerpo genéticamente modificado para reconocer la Miostatina y neutralizarla por la compañía farmacéutica de New-Jersey Wyeth. El inhibidor se llama MYO-029 y está actualmente en fase experimental en humanos. Algunos atletas, impacientes por conseguir tales drogas, buscan por Internet, donde se están vendiendo bloqueadores de la Miostatina falsos.
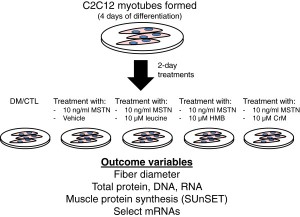
El tratamiento fué llevado a cabo por cuatro grupos, los cuales fuerón estudiados de la siguiente manera:
1) 3 veces por día mM Leu 10,
2) 3 veces por día HMB 10 mM,
3) 3x día mM Creatina 10, sólo
4) DM, grupo control.
Después del tratamiento, células como la proteína total, el contenido de ADN, el contenido de ARN, la síntesis de proteínas musculares (MPS) y el diámetro de la fibra, se analizarón exhaustivamente. Se analizarón los tratamientos por separado para los patrones de expresión de mRNA de los genes relacionados con la miostatina (Akirin-1 / Mighty, Notch-1, Esquí, MyoD), así como atrogenes (MuRF-1, y MAFbx / atrogina-1)
Resultados
NTCR diámetro de la fibra disminuyó aproximadamente un 30% en comparación con DM / miotubos CTL (p <0,001). Leu, HMB y Creatina podrían haber sido los responsables de prevenir la atrofia inducida por NTCR. NTCR no disminuyó los niveles de MPS en comparación con DM / miotubos CTL, pero el tratamiento NTCR disminuyó la expresión de ARNm de Akirin-1 / Mighty en un 27% (p <0,001) y MyoD en un 26% (p <0,01) en comparación con DM / miotubos CTL . Sorprendentemente, NTCR + Leu y NTCR + HMB miotubos tenían niveles Akirin-1 / Mighty y MyoD ARNm similares en comparación con DM / miotubos CTL.
Conclusión
Los investigadores demuestran como la administración de L-leucina, HMB y creatina monohidrato puede revertir la atrofia inducida por miostatina en los miotubos; Potencialmente, esto resulta de la acción independiente de la modulación de cada ingrediente Akirin-1 / Mighty expresión de ARNm. Además, los resultados sugieren que, a pesar de los tratamientos NTCR, el tratamiento de monohidrato de creatina fué capaz de regular la ARNm Akirin-1 / Mighty lo que conduce a un efecto hipertrófico claramente independiente de la síntesis de proteína muscular. Futuro en estudios in vivo debe seguir examinando cómo leucina, HMB, y / o monohidrato de creatina de forma independiente o sinérgica afectan en la expresión genética de Akirin-1 / Mighty .
BIBLIOGRAFIA
- Jackman RW, Kandarian SC:The molecular basis of skeletal muscle atrophy.
Am J Physiol Cell Physiol 2004, 287(4):C834-C843.
- Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K:Transcriptional pathways associated with skeletal muscle disuse atrophy in humans.
Physiol Genomics 2007, 31(3):510-520.
- Elkina Y, von Haehling S, Anker SD, Springer J:The role of myostatin in muscle wasting: an overview.
J Cachexia Sarcopenia Muscle 2011, 2(3):143-151.
- Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA:Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men.
J Appl Physiol (1985) 2009, 106(5):1720-1729.
- Goodman CA, McNally RM, Hoffmann FM, Hornberger TA:Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo.
Mol Endocrinol 2013, 27(11):1946-1957.
- McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R:Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism.
J Cell Physiol 2006, 209(2):501-514.
- Joulia-Ekaza D, Cabello G:Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects.
Exp Cell Res 2006, 312(13):2401-2414.
- Wu M, Fannin J, Rice KM, Wang B, Blough ER:Effect of aging on cellular mechanotransduction.
Ageing Res Rev 2011, 10(1):1-15.
- Dalbo VJ, Roberts MD, Sunderland KL, Poole CN, Stout JR, Beck TW, Bemben M, Kerksick CM:Acute loading and aging effects on myostatin pathway biomarkers in human skeletal muscle after three sequential bouts of resistance exercise.
J Gerontol A Biol Sci Med Sci 2011, 66(8):855-865.
- Sakuma K, Yamaguchi A:Molecular mechanisms in aging and current strategies to counteract sarcopenia.
Curr Aging Sci 2010, 3(2):90-101.
- Leger B, Derave W, De Bock K, Hespel P, Russell AP:Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation.
Rejuvenation Res 2008, 11(1):163-175B.
- Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, Sharma M:Antagonism of myostatin enhances muscle regeneration during sarcopenia.
Mol Ther 2007, 15(8):1463-1470.
- Tobin JF, Celeste AJ:Myostatin, a negative regulator of muscle mass: implications for muscle degenerative diseases.
Curr Opin Pharmacol 2005, 5(3):328-332.
- Kawada S, Tachi C, Ishii N:Content and localization of myostatin in mouse skeletal muscles during aging, mechanical unloading and reloading.
J Muscle Res Cell Motil 2001, 22(8):627-633.
- Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF:Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting.
J Nutr Health Aging 2002, 6(5):343-348.
- Louis E, Raue U, Yang Y, Jemiolo B, Trappe S:Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle.
J Appl Physiol (1985) 2007, 103(5):1744-1751.
- Dalbo VJ, Roberts MD, Hassell S, Kerksick CM:Effects of pre-exercise feeding on serum hormone concentrations and biomarkers of myostatin and ubiquitin proteasome pathway activity.
Eur J Nutr 2013, 52(2):477-487.
- Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL:Myostatin expression during human muscle hypertrophy and subsequent atrophy: increased myostatin with detraining.
Scand J Med Sci Sports 2011, 21(2):215-223.
- Anthony JC, Anthony TG, Kimball SR, Jefferson LS:Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine.
J Nutr 2001, 131(3):856S-860S.
- Stipanuk MH:Leucine and protein synthesis: mTOR and beyond.
Nutr Rev 2007, 65(3):122-129.
- Haegens A, Schols AM, van Essen AL, van Loon LJ, Langen RC:Leucine induces myofibrillar protein accretion in cultured skeletal muscle through mTOR dependent and -independent control of myosin heavy chain mRNA levels.
Mol Nutr Food Res 2012, 56(5):741-752.
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J, Attaix D:A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle.
J Physiol 2005, 569(Pt 2):489-499.
- Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr, Connelly AS, Abumrad N:Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training.
J Appl Physiol (1985) 1996, 81(5):2095-2104.
- Slater GJ, Jenkins D:Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength.
Sports Med 2000, 30(2):105-116.
- Jowko E, Ostaszewski P, Jank M, Sacharuk J, Zieniewicz A, Wilczak J, Nissen S:Creatine and beta-hydroxy-beta-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program.
Nutrition 2001, 17(7–8):558-566.
- Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ:Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli.
Am J Physiol Endocrinol Metab 2007, 293(4):E923-E931.
- Wilson GJ, Wilson JM, Manninen AH:Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review.
Nutr Metab (Lond) 2008, 5:1.
- Wilson JM, Lowery RP, Joy JM, Walters JA, Baier SM, Fuller JC Jr, Stout JR, Norton LE, Sikorski EM, Wilson SM, Duncan NM, Zanchi NE, Rathmacher J:beta-Hydroxy-beta-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men.
Br J Nutr 2013, 110(3):538-544.
- Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M:Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle.
J Appl Physiol (1985) 2008, 104(2):371-378.
- Earnest CP, Snell PG, Rodriguez R, Almada AL, Mitchell TL:The effect of creatine monohydrate ingestion on anaerobic power indices, muscular strength and body composition.
Acta Physiol Scand 1995, 153(2):207-209.
- Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL:Muscle creatine loading in men.
J Appl Physiol (1985) 1996, 81(1):232-237.
- Clarkson PM, Rawson ES:Nutritional supplements to increase muscle mass.
Crit Rev Food Sci Nutr 1999, 39(4):317-328.
- Francaux M, Poortmans JR:Effects of training and creatine supplement on muscle strength and body mass.
Eur J Appl Physiol Occup Physiol 1999, 80(2):165-168.
- Becque MD, Lochmann JD, Melrose DR:Effects of oral creatine supplementation on muscular strength and body composition.
Med Sci Sports Exerc 2000, 32(3):654-658.
- Kreider RB:Effects of creatine supplementation on performance and training adaptations.
Mol Cell Biochem 2003, 244(1–2):89-94.
- Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, Brachat S, Rivet H, Koelbing C, Morvan F, Hatakeyama S, Glass DJ:An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy.
Mol Cell Biol 2014, 34(4):606-618.
- Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ:Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size.
Am J Physiol Cell Physiol 2009, 296(6):C1258-C1270.
- Deldicque L, Theisen D, Bertrand L, Hespel P, Hue L, Francaux M:Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways.
Am J Physiol Cell Physiol 2007, 293(4):C1263-C1271.
- Smith HJ, Wyke SM, Tisdale MJ:Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate.
Cancer Res 2004, 64(23):8731-8735.
- Goodman CA, Hornberger TA:Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques?
Exerc Sport Sci Rev 2013, 41(2):107-115.
- Nader GA, Hornberger TA, Esser KA:Translational control: implications for skeletal muscle hypertrophy.
Clin Orthop Relat Res 2002, (403 Suppl):S178-S187.
- Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH Jr, Kull FC Jr, Gonzalez-Cadavid N:Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells.
Am J Physiol Endocrinol Metab 2001, 280(2):E221-E228.
- Rios R, Carneiro I, Arce VM, Devesa J:Myostatin is an inhibitor of myogenic differentiation.
Am J Physiol Cell Physiol 2002, 282(5):C993-C999.
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R:Myostatin negatively regulates satellite cell activation and self-renewal.
J Cell Biol 2003, 162(6):1135-1147.
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J:Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal.
J Nutr 2002, 132(1):95-100.
- Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Nascimento CM O d, de Mello MT, Tufik S, Santos RV:beta-Hydroxy-beta-methylbutyrate (HMbeta) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway.
Nutr Metab (Lond) 2011, 8(1):11.
- Talvas J, Obled A, Fafournoux P, Mordier S:Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes.
J Nutr 2006, 136(6):1466-1471.
- Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA:Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis.
J Appl Physiol (1985) 2001, 91(3):1041-1047.
- MacKenzie MG, Hamilton DL, Pepin M, Patton A, Baar K:Inhibition of myostatin signaling through Notch activation following acute resistance exercise.
PLoS One 2013, 8(7):e68743.
- Macqueen DJ, Kristjansson BK, Johnston IA:Salmonid genomes have a remarkably expanded akirin family, coexpressed with genes from conserved pathways governing skeletal muscle growth and catabolism.
Physiol Genomics 2010, 42(1):134-148.
- Marshall A, Salerno MS, Thomas M, Davies T, Berry C, Dyer K, Bracegirdle J, Watson T, Dziadek M, Kambadur R, Bower R, Sharma M:Mighty is a novel promyogenic factor in skeletal myogenesis.
Exp Cell Res 2008, 314(5):1013-1029.
- Salerno MS, Dyer K, Bracegirdle J, Platt L, Thomas M, Siriett V, Kambadur R, Sharma M:Akirin1 (Mighty), a novel promyogenic factor regulates muscle regeneration and cell chemotaxis.
Exp Cell Res 2009, 315(12):2012-2021.
- Dong Y, Pan JS, Zhang L:Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction.
PLoS One 2013, 8(3):e58554.
- Shelmadine B, Cooke M, Buford T, Hudson G, Redd L, Leutholtz B, Willoughby DS:Effects of 28 days of resistance exercise and consuming a commercially available pre-workout supplement, NO-Shotgun(R), on body composition, muscle strength and mass, markers of satellite cell activation, and clinical safety markers in males.
J Int Soc Sports Nutr 2009, 6:16.
- Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O:Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways.
Biochim Biophys Acta 2009, 1793(5):755-763.
- Aoki MS, Lima WP, Miyabara EH, Gouveia CH, Moriscot AS:Deleteriuos effects of immobilization upon rat skeletal muscle: role of creatine supplementation.
Clin Nutr 2004, 23(5):1176-1183.
- Johnston AP, Burke DG, MacNeil LG, Candow DG:Effect of creatine supplementation during cast-induced immobilization on the preservation of muscle mass, strength, and endurance.
J Strength Cond Res 2009, 23(1):116-120.
- Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M:Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training.
J Physiol 2006, 573(Pt 2):525-534.
- Willoughby DS, Rosene JM:Effects of oral creatine and resistance training on myogenic regulatory factor expression.
Med Sci Sports Exerc 2003, 35(6):923-929.
- Hespel P, Op’t Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA:Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans.
J Physiol 2001, 536(Pt 2):625-633.
- Haussinger D, Roth E, Lang F, Gerok W:Cellular hydration state: an important determinant of protein catabolism in health and disease.
<
p style=»font-weight:inherit;font-style:inherit;»>Lancet 1993, 341(8856):1330-1332.